Colloids consist of particles dispersed in a medium, forming a stable mixture with small particle size and no settling over time. Suspensions involve larger particles that tend to settle due to gravity.
TL;DR Colloid Vs. Suspension
Colloids are mixtures with small particles suspended in a medium, remaining stable over time. They exhibit unique properties, scatter light, and find applications in various industries.
Suspensions, have larger visible particles that tend to settle due to gravity. They require shaking or stirring for even distribution. Both are heterogeneous mixtures but differ in particle size and behavior.
Colloids
Colloids are mixtures containing tiny particles, larger than molecules but smaller than visible to the naked eye, dispersed in a medium. They exhibit unique properties, remaining stable and not settling over time.
Colloids can scatter light, creating an opalescent appearance. They come in different forms such as aerosols, emulsions, and foams. Colloids find applications in various industries, from cosmetics and food production to pharmaceuticals and materials science.
Suspensions
Suspensions are heterogeneous mixtures consisting of larger particles or droplets that are visible to the naked eye, dispersed in a liquid or gas medium. Unlike colloids, suspensions do not remain stable over time and tend to settle due to gravity.
They require agitation or stirring to maintain even distribution. Common examples include muddy water, orange juice with pulp, and fog.
Suspensions are used in various industries, such as pharmaceuticals, cosmetics, and paints, where the even dispersion of particles is necessary for their applications.
Colloid Vs. Suspension – Key differences
| Aspect | Colloids | Suspensions |
|---|---|---|
| Particle size | Intermediate (larger than molecules) | Larger (visible to the naked eye) |
| Stability | Stable over time (do not settle) | Unstable (tend to settle over time) |
| Behavior with light | Scatter light (Tyndall effect) | Do not scatter light significantly |
| Examples | Aerosols (smoke), emulsions (milk), foams | Muddy water, orange juice with pulp, fog |
| Agitation | Do not require agitation for stability | Require agitation for even distribution |
| Nature | Homogeneous appearance with small particles | Heterogeneous appearance with visible particles |
| Applications | Widely used in cosmetics, food, materials science | Found in pharmaceuticals, paints, and other industries for particle dispersion |
Stable and Unstable Colloids
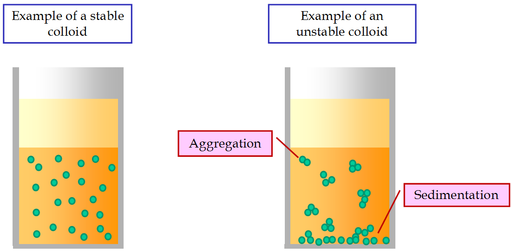
Stable and unstable colloids refer to the behavior of the colloidal particles within the dispersion. It relates to whether the particles remain dispersed and do not settle or aggregate over time, or if they tend to aggregate and settle, leading to the breakdown of the colloid.
Stable Colloids: In stable colloids, the dispersed particles remain evenly distributed within the medium, and the colloid maintains its homogeneous appearance over an extended period.
Unstable Colloids: Unstable colloids are colloidal systems in which the particles tend to aggregate and settle over time, resulting in a phase separation and a heterogeneous appearance.
Types of Colloids
- Sol: A colloid in which solid particles are dispersed in a liquid medium. Examples include ink, blood plasma, and some types of paint.
- Gel: Gels are colloids with a solid-like consistency due to a three-dimensional network of particles within a liquid medium. Jellies, custards, and some cosmetics are examples of gels.
- Emulsion: Emulsions are colloids where tiny droplets of one immiscible liquid are dispersed throughout another immiscible liquid. Examples include milk, mayonnaise, and certain types of salad dressings.
- Foam: Foams consist of gas bubbles trapped in a liquid or solid matrix. Whipped cream, meringue, and sponge cake batter are examples of foams.
- Aerosol: Aerosols are colloids where small particles or droplets are suspended in a gas medium. Examples include smoke, fog, and certain sprays.
- Sols in Gas: Some colloids have solid particles dispersed in a gas medium. For example, smoke is a colloid where solid particles are suspended in the air.
Types of Suspensions
Suspensions can be categorized into different types based on the nature of the dispersed particles and the medium in which they are suspended.
- Solid-Liquid Suspension: In this type, solid particles are dispersed in a liquid medium. Examples include muddy water, clay suspended in water, and sand suspended in oil.
- Liquid-Gas Suspension: This type involves tiny liquid droplets dispersed in a gaseous medium. Fog is a classic example where water droplets disperse through the air, creating misty conditions.
- Gas-Solid Suspension: In gas-solid suspensions, solid particles are dispersed within a gaseous medium. Smoke is a common example, where tiny carbon particles are suspended in the air.
- Emulsion: Emulsions are a specific type of liquid-liquid suspension, where two immiscible liquids form a stable mixture due to the presence of an emulsifying agent. Mayonnaise and milk are examples of emulsions.
- Foam: Foam is a type of suspension where gas bubbles are dispersed throughout a liquid or solid matrix. Whipped cream, soap bubbles, and certain types of insulation foam are examples of foams.
Image Credits
Featured Image By – NASA, Public domain, via Wikimedia Commons
Image 1 By – SunKart at English Wikipedia, CC BY 3.0, via Wikimedia Commons








