Xenon produces brighter and whiter light, is more energy-efficient, and has a longer lifespan than halogen. Xenon is a gas, while halogen is a filament.
TL;DR Xenon Vs. Halogen
Xenon is a noble gas known for its stability, low reactivity, and unique ability to produce bright light in various fields such as lighting technology and medicine. Halogen refers to a group of highly reactive elements that are commonly used in industry for their disinfectant properties.
While xenon is primarily used in specialized areas like high-intensity discharge lamps and medical imaging devices, halogens find extensive use in everyday products such as household cleaning agents, water treatment systems, and even pharmaceuticals.
What is Xenon?
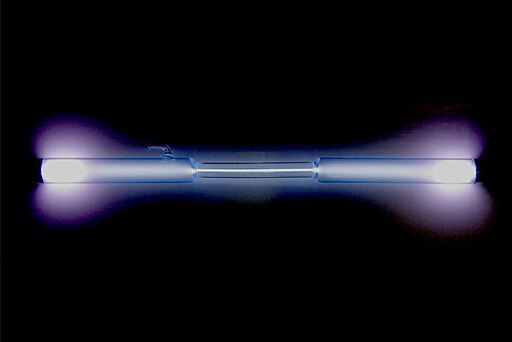
Xenon is a chemical element with the symbol Xe and atomic number 54. As a noble gas, it belongs to Group 18 of the periodic table. Xenon is colorless, odorless, and tasteless, and it occurs in trace amounts in Earth’s atmosphere.
Its name is derived from the Greek word “xenos,” meaning stranger, reflecting its relative scarcity. Xenon is notable for its applications in lighting, particularly in xenon headlights, where its unique properties produce bright, white light.
Beyond lighting, xenon has various uses, including in medical imaging equipment like xenon CT scanners. Its stable nature and high density make it suitable for certain types of lasers, and it is employed in experimental plasma research.
Despite being a minor component of the atmosphere, xenon’s rarity and distinct characteristics make it valuable in technological and industrial applications. In some futuristic concepts, xenon has been considered for use in ion propulsion systems for spacecraft due to its ability to be easily ionized.
What is Halogen?

Halogens are a group of chemical elements belonging to Group 17 (VIIA) of the periodic table, including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They share similar properties, characterized by high reactivity and a tendency to form salts when combined with metals. The name “halogen” is derived from the Greek words “halos,” meaning salt, and “genes,” meaning forming.
In everyday applications, halogen most commonly refers to halogen lamps or bulbs. These lighting sources use a tungsten filament encased in a small amount of a halogen gas (such as iodine or bromine) within the bulb. The halogen cycle allows for the recycling of tungsten vapor back onto the filament, extending the bulb’s lifespan and maintaining a consistent light output.
Aside from lighting, halogens find use in various industries and applications. Chlorine, for instance, is employed in water treatment, and fluorine is used in the production of pharmaceuticals and in the electronics industry. Despite their reactivity, halogens play crucial roles in both natural and industrial processes.
Xenon Vs. Halogen – Key differences
| Characteristic | Xenon | Halogen |
|---|---|---|
| Type | Noble Gas | Group 17 Elements (Fluorine, Chlorine, Bromine, Iodine, Astatine) |
| State at Room Temp. | Gas | Fluorine and Chlorine: Gas; Bromine: Liquid; Iodine and Astatine: Solid |
| Occurrence | Trace amounts in Earth's atmosphere | Found in nature, e.g., as sodium chloride (table salt) |
| Lighting Applications | Xenon headlights are common in cars | Halogen bulbs widely used in general lighting |
| Color of Light | Bright, white light | Yellowish light |
| Efficiency | More energy-efficient | Less energy-efficient |
| Lifespan | Longer lifespan | Shorter lifespan |
| Chemical Properties | Inert, stable | Reactive, forms salts with metals |
| Uses | Lighting, medical imaging, lasers, experimental plasma research | General lighting, water treatment (e.g., chlorine), electronics (e.g., fluorine) |
Physical and Chemical Properties of Xenon
Physical Properties of Xenon
- State at Room Temperature: Gas
- Atomic Number: 54
- Atomic Mass: 131.293 u
- Density: 0.005887 g/cm³ (at 0 °C and 1 atm)
- Melting Point: -111.9 °C
- Boiling Point: -108.1 °C
- Color: Colorless
- Odor: Odorless
- Solubility: Insoluble in water
- Phase: Gas at room temperature and pressure
Chemical Properties of Xenon
- Chemical Symbol: Xe
- Group: 18 (Noble Gases)
- Reactivity: Generally inert; undergoes few chemical reactions
- Ionization Energy: Relatively high due to its noble gas configuration
- Electronegativity: Low
- Isotopes: Naturally occurring xenon consists of several isotopes, with Xe-129 and Xe-131 being the most abundant.
- Stability: Chemically stable and unreactive under normal conditions.
- Compounds: Xenon can form compounds, known as xenon compounds or xenon fluorides, under specific conditions. These are relatively unstable and unusual compared to compounds of other elements.
Xenon’s unique properties make it valuable in various applications, including lighting, medical imaging, lasers, and experimental plasma research. Its stability and relative inertness contribute to its usefulness in specialized technologies.
Physical and Chemical Properties of Halogen
Physical Properties of Halogens
- State at Room Temperature: Fluorine (F) and Chlorine (Cl) are gases; Bromine (Br) is a liquid; Iodine (I) and Astatine (At) are solids.
- Density: Varies – fluorine and chlorine are low-density gases; bromine is a dense liquid; iodine and astatine are relatively high-density solids.
- Melting Points: Vary – fluorine and chlorine have very low melting points; bromine has a low melting point for a liquid; iodine and astatine have higher melting points for solids.
- Boiling Points: Vary – fluorine and chlorine have very low boiling points; bromine has a low boiling point for a liquid; iodine and astatine have higher boiling points for solids.
- Color: Fluorine and chlorine are pale yellow and greenish-yellow gases, respectively; bromine is a reddish-brown liquid; iodine is a violet solid; astatine’s appearance is uncertain but is expected to be metallic.
Chemical Properties of Halogens
- Chemical Symbol: F (Fluorine), Cl (Chlorine), Br (Bromine), I (Iodine), At (Astatine)
Group: 17 (Halogens) - Reactivity: Decreases down the group – fluorine is highly reactive, while astatine is expected to be less reactive.
- Electronegativity: High electronegativity, making them strong oxidizing agents.
Ionization Energy: Decreases down the group. - Valence Electrons: Seven valence electrons, making them likely to gain an electron to achieve a stable electron configuration.
- Compounds: Form halides (salts) when combined with metals; can also form covalent compounds.
Halogens exhibit diverse chemical behaviors due to their varying physical states and reactivity. They play essential roles in various applications, including disinfection (chlorine), pharmaceuticals (iodine), and organic synthesis
Applications of Halogen in Industry
Halogens, including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), have various applications in the industry due to their unique chemical properties. Here are some notable applications:
Water Treatment:
- Chlorine: Widely used for disinfection of water in municipal water treatment plants and swimming pools.
Chemical Synthesis:
- Chlorine and Bromine: Used in the synthesis of a wide range of organic compounds, including plastics, solvents, and pharmaceuticals.
Pharmaceuticals:
- Iodine: Used in the production of pharmaceuticals, particularly as an antiseptic in medical applications.
Plastics and Polymers:
- Chlorine: Essential in the production of PVC (polyvinyl chloride), a widely used plastic.
Solvent Production:
- Chlorine: Used in the production of various solvents, including chloroform and carbon tetrachloride.
Flame Retardants:
- Bromine: Incorporated into flame retardants for plastics and textiles to improve fire resistance.
Agricultural Chemicals:
- Chlorine and Bromine: Used in the production of pesticides and herbicides for agricultural applications.
Electronics:
- Fluorine: Used in the electronics industry, especially in the production of high-performance polymers and as a cleaning agent for semiconductor manufacturing.
Oil and Gas Industry:
- Chlorine and Bromine: Employed in various processes, such as drilling fluids and well completion fluids.
Photography:
- Iodine: Historically used in the production of photographic chemicals.
Applications of Xenon in Industry
Xenon, though relatively rare in Earth’s atmosphere, has several unique properties that make it valuable in various industrial applications. Some notable uses of xenon in industry include:
Lighting:
- Xenon Headlights: Used in automotive lighting due to its bright and white light, which enhances visibility. Xenon lamps are also utilized in high-intensity discharge (HID) lamps for general lighting applications.
Medical Imaging:
- Xenon CT Scanners: Xenon gas is employed as a contrast agent in computed tomography (CT) scans to enhance the visibility of blood vessels and improve imaging quality.
Laser Technology:
- Xenon Flash Lamps: Used as a source of intense, short-duration light in flash lamps for lasers, particularly in applications like medical lasers and cosmetic procedures.
Satellite Propulsion:
- Ion Propulsion Systems: Xenon is used as a propellant in ion propulsion systems for satellites and spacecraft due to its relatively high atomic mass, allowing for efficient acceleration.
Experimental Plasma Research:
- Plasma Physics Experiments: Xenon is used in certain experimental setups for studying plasma physics and other scientific research.
Nuclear Reactor Control:
- Xenon-135 Control Rods: Xenon-135, a radioactive isotope of xenon, can be used in control rods for regulating nuclear reactors.
Bubble Chambers:
- Particle Physics Research: Xenon is sometimes used in particle physics experiments, such as in bubble chambers, to detect and study subatomic particles.
Space Exploration:
- Deep Space Missions: Xenon is considered for use as a propellant in deep space missions due to its favorable properties for ion propulsion systems.
Image Credits
Featured Image By – Jörn Schimmelmann from Pixabay
Image 1 By – Alchemist-hp (talk) (www.pse-mendelejew.de), FAL, via Wikimedia Commons
Image 2 By – Nguyễn Tự Khanh from Pixabay








