Carbon monoxide is a deadly gas that forms when there is incomplete combustion of fossil fuels. It is odorless, colorless, and tasteless, Carbon dioxide is a naturally occurring gas that we exhale with each breath. It plays a vital role in maintaining the Earth’s temperature by trapping heat from the sun – an effect known as the greenhouse effect.
TL;DR Carbon Monoxide Vs. Carbon Dioxide
Carbon monoxide (CO) has one carbon atom and one oxygen atom, is toxic, and mainly produced by incomplete combustion. Carbon dioxide (CO2) has one carbon atom and two oxygen atoms, is non-toxic in small quantities,
It is also a natural part of the atmosphere but contributes to climate change when produced in excess.
Carbon Monoxide

Carbon monoxide, often referred to as CO, is a colorless and odorless gas. It is composed of one carbon atom bonded with one oxygen atom. This potent gas is produced through the incomplete combustion of fossil fuels such as gasoline, coal, and natural gas. We commonly encounter carbon monoxide in our daily lives due to its presence in exhaust fumes from vehicles and the burning of wood or other materials.
Carbon Dioxide
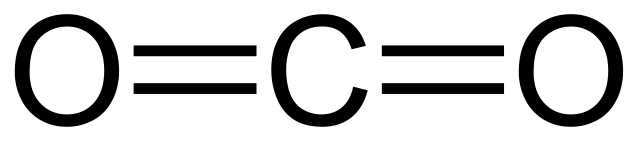
Carbon dioxide is a colorless and odorless gas that plays a crucial role in our atmosphere. It is composed of one carbon atom bonded to two oxygen atoms, hence its chemical formula CO2.
Carbon Monoxide Vs. Carbon Dioxide – Key differences
| Carbon Monoxide (CO) | Carbon Dioxide (CO2) | |
|---|---|---|
| Chemical Formula | CO | CO2 |
| Composition | Contains one carbon atom and one oxygen atom. | Comprises one carbon atom and two oxygen atoms. |
| Molecular Weight | 28.01 g/mol | 44.01 g/mol |
| Toxicity | Highly toxic and can be life-threatening in high concentrations. It binds strongly to hemoglobin, reducing oxygen-carrying capacity in the blood. | Non-toxic in small quantities but can be harmful in high concentrations, causing difficulty in breathing and asphyxiation in confined spaces. |
| Natural Occurrence | Can be produced by incomplete combustion of carbon-containing fuels like gasoline, wood, and coal. Also present in vehicle exhaust and cigarette smoke. | Produced by respiration in living organisms, combustion processes, and natural processes like volcanic eruptions and decomposition. It is a vital component of the Earth's atmosphere. |
| Color and Odor | Colorless, odorless, and tasteless gas, making it hard to detect without specialized equipment. | Colorless and odorless gas, but when present in high concentrations, it can cause a slightly acidic taste and a feeling of breathlessness. |
| Environmental Impact | Considered a major air pollutant due to its toxicity and role in contributing to air pollution and smog. | A significant greenhouse gas responsible for trapping heat in the Earth's atmosphere, contributing to global warming and climate change. |
| Health Effects | Can lead to headaches, dizziness, confusion, and even death when inhaled in large amounts. | Breathing in high concentrations can cause rapid breathing, dizziness, and can be harmful to individuals with respiratory conditions. However, it is essential for normal respiration in moderate concentrations. |
| Fire Characteristics | Highly flammable and can support combustion. | Not flammable and does not support combustion. |
| Detection Methods | Requires specialized detectors to detect its presence, especially in confined spaces. | Not typically detected without specialized equipment, but its presence can be inferred based on other indicators, such as elevated levels in enclosed environments. |
Applications of Carbon Monoxide in daily life
Carbon monoxide (CO) is a highly toxic gas, and it is essential to understand that its use in daily life is limited due to its hazardous nature.
However, there are a few practical applications of carbon monoxide in controlled and specialized settings:
- Industrial Processes: Production of chemicals like acetic acid, methanol, and hydrogen. It is also used in the extraction of certain metals from their ores.
- Chemical Synthesis: CO is an essential building block in the synthesis of various organic compounds, including alcohols, aldehydes, and carboxylic acids.
- Hydrogen Production: CO is involved in some methods of hydrogen production, such as water-gas shift reaction.
- Metal Carbonyls: Some metal carbonyls, formed by the reaction of metals with carbon monoxide, are utilized in industrial applications as catalysts and as a source of metal nanoparticles.
- Lab Research and Testing: In controlled laboratory settings, carbon monoxide is used to study its properties and reactions, as well as to explore its potential applications.
It is essential to handle carbon monoxide with extreme caution due to its highly toxic nature. In daily life, carbon monoxide is primarily known as a dangerous byproduct of incomplete combustion from sources like vehicle engines, stoves, furnaces, and fireplaces. Exposure to high levels of carbon monoxide can lead to carbon monoxide poisoning, which can be life-threatening. To prevent accidental exposure, it is crucial to have carbon monoxide detectors in homes and workplaces, especially in areas with fuel-burning appliances.
Applications of Carbon Dioxide in daily life
Carbon dioxide (CO2) is a naturally occurring gas that has various practical applications in daily life. Some of the key applications include:
- Carbonated Beverages: CO2 is widely used in the beverage industry to carbonate soft drinks, soda water, sparkling water.
- Food Preservation: CO2 is used as a preservative to extend the shelf life of certain products. It helps to slow down the growth of bacteria and fungi.
- Fire Extinguishers: Carbon dioxide is used in fire extinguishers to displace oxygen and smother fires.
- Welding and Metal Fabrication: CO2 is employed as a shielding gas in welding processes like MIG (Metal Inert Gas) welding.
- Greenhouses and Indoor Plant Cultivation: CO2 is supplied to greenhouses and indoor gardens to enhance plant growth. Higher levels of CO2 can promote photosynthesis and increase crop yields.
CO2 is also a significant greenhouse gas responsible for global warming and climate change. Proper management of carbon dioxide emissions is crucial to mitigate its impact on the environment.
Image Credits
Featured Image By – Jorge Stolfi, Public domain, via Wikimedia Commons
Image 1 By – Radekkie, Public domain, via Wikimedia Commons
Image 2 By – Jü, Public domain, via Wikimedia Commons